New CIPA Electrolyte Achieves Long-Lifetime Lithium Metal Pouch Cells: 505.9 Wh/kg Density with 91% Energy Retention After 130 Cycles
August 14 News: Although lithium metal
batteries theoretically have an energy density exceeding 500 Wh/kg, their commercialization is significantly limited due to their very limited cycle life. This limitation is mainly due to the poor stability of the electrolyte and electrode interfaces, with traditional electrolytes struggling to be compatible with lithium metal anodes and high-voltage cathodes. Therefore, designing an electrolyte that provides high interface stability at both the anode and cathode is key to achieving high-energy-density lithium metal batteries.
The Chinese Academy of Sciences has announced that Wang Xuefeng, a distinguished researcher at the Institute of Physics / National Center for Condensed Matter Physics in Beijing, in collaboration with researchers from Peking University, the University of Science and Technology of China, and Soochow University, has proposed a new type of Compact Ion-Pair Aggregate (CIPA) electrolyte. This electrolyte enables the development of long-lifetime lithium metal pouch cells under thin electrolyte conditions.
According to official information, this CIPA electrolyte, which features a unique nanoscale solvation structure, has been used to assemble lithium metal pouch cells with a high-nickel cathode (LiNi₀.₉₀₅Co₀.₀₆Mn₀.₀₃₅O₂) achieving an energy density of 505.9 Wh/kg. The energy retention rate of these cells is 91% after 130 cycles.
The related research results have been published in the journal *Nature Energy* under the title Towards Long-Life 500 Wh/kg Lithium Metal Pouch Cells via Compact Ion-Pair Aggregate Electrolytes.
▲ Interface characterization of lithium metal anode
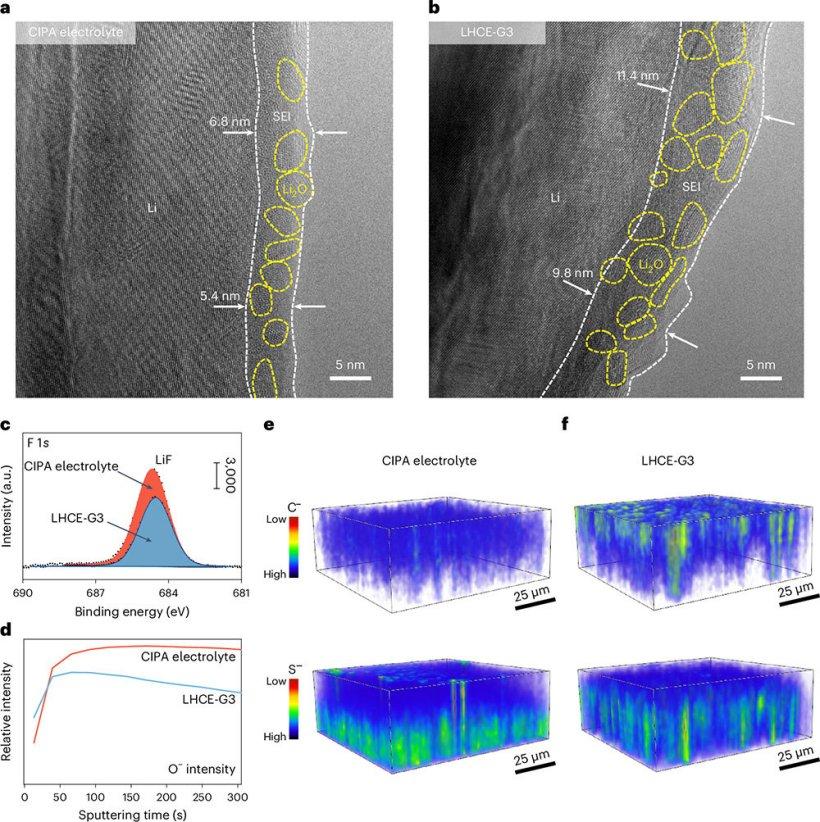
The study employed cryo-transmission electron microscopy in combination with a series of advanced surface analysis techniques to investigate the structure, composition, and spatial distribution of the solid electrolyte interphase (SEI) on the electrolyte interface.
In terms of structure, the CIPA electrolyte forms a thin and uniform SEI film on the lithium metal anode surface. This film has an average thickness of about 6.2 nm, which is thinner than the SEI films formed with high-concentration electrolytes. Both electrolytes produce SEI films with an organic-inorganic composite structure, but the SEI film formed with the CIPA electrolyte exhibits higher uniformity in the size and distribution of Li2O nanoparticles.
Regarding composition, the SEI formed with the LHCE-G3 electrolyte contains less LiF and shows stronger C- and O-signals compared to the SEI formed with the CIPA electrolyte, indicating reduced decomposition of FSI⁻ anions and more severe solvent decomposition.
In terms of spatial distribution, the CIPA electrolyte-derived SEI film shows a more uniform distribution of C- and S-elements from inside to outside. In contrast, the LHCE-G3 electrolyte SEI film shows a prominent C-signal on the outer layer and a concentrated S-signal on the inner layer. This uneven reduction behavior of the LHCE-G3 electrolyte can lead to localized lithium deposition and dendrite formation.
The research findings on interface characterization reveal that FSI⁻ anions in the CIPA electrolyte are rapidly reduced through a collective electronic transfer mechanism, forming an SEI film rich in LiF and Li2O with low organic content. This stable SEI layer provides effective protection for the lithium metal anode, preventing further side reactions and thus enhancing the cycle stability and safety of lithium metal batteries.
Next:The 4680 Battery: Technical Specifications Comparison with Traditional Batteries, Market Trends, and Future Potential
Previous:Understanding the Advantages and Disadvantages of Lithium Iron Phosphate (LiFePO4) Batteries
